V-Safe Part 10: Federal Judge Orders CDC to Make Public 7.8 Million V-safe Free-Text Entries Within 12 Months
Tenth part of an incredible story that shows just how broken our public “health” apparatus is: very, very broken.
I am pleased to share that United States District Judge Matthew J. Kacsmaryk has issued a decision and order requiring CDC to release the 7.8 million free-text entries from CDC’s COVID-19 V-Safe program to the public. These are entries sent to CDC from roughly 10 million V-safe users, typically detailing injuries following receipt of a COVID-19 vaccine.
As many of you know, in our prior case, we obtained the V-safe check-the-box data and, and now through this case, the V-safe free-text data will also be made available to the public. Why would CDC want to conceal the free-text entries from the public? Just check out one example of a free-text entry we did obtain and the answer may become plain, as covered in my prior post “CDC Designs V-Safe to Assure Harms Are Hidden in Free-Text Fields…”
Here are excerpts from the incredible decision, which you can read in full here:
As COVID-19 spread, the federal government collaborated and cooperated with foreign governments and non-governmental humanitarian organizations, private companies, and the media to enable and incentivize widespread vaccination.…
The government promoted vaccination — directly through mandates or indirectly through policies, privileges, and messaging campaigns. Many employers required vaccination via various workplace rules, regulations, and policies. … Societal reality hinged on vaccination status — from school attendance to family vacations. By early 2023, more than 5.5 billion people (about 72.3 percent of the world population) had received a dose of a COVID-19 vaccine, including more than 270 million Americans. Defendants have consistently asserted that “COVID-19 vaccines are safe and effective,” “recommends everyone ages 6 months and older get an updated COVID-19 vaccine,” and added the COVID-19 vaccine to standard Child and Adolescent Immunization Schedule. …
While “Trust the Science” became something of a national slogan, the American public’s trust in science and scientists are at an all-time low. It is with this background that Plaintiff aims to further the ideals pledged by the Biden-Harris administration: to “Promote trust, transparency, common purpose, and accountability in our government” by making available for public access — and particularly for independent scientific and medical research — all of the relevant health data collected through the V-safe program. As the check-the-box data has already been released, it is the free-text response data Plaintiff seeks….
The development and distribution of the COVID-19 vaccine was one of the greatest endeavors in recent history. Predictably, the American public now seeks access to COVID-related papers to ensure that relevant government policies were — and still are — supported and justified by the available data. That is precisely what FOIA contemplates and facilitates.
It is also what Defendants expected and envisioned for V-safe — at least initially. V-safe protocol intended that “[a] final data set . . . with deidentified data will be made available for external data requests or through Freedom of Information Act (FOIA) requests.” V-Safe Protocol: April 18, 2022 ... However, Defendants now argue … “... the V-safe application collected considerably more data and was operational for a longer period than initially anticipated.” … The simple reason Defendants denied Plaintiff’s production request is the 7.8 million free-text response entries are allegedly too numerous for the agency’s limited resources.… While the burden to produce the requested free-text responses may be heavy, this Court does not find that it is unreasonable….
Instead, this Court finds that production is not unreasonably burdensome for at least four reasons: the requested records are not so voluminous; only a small percent of records will require any redaction; the redaction process is largely straightforward and capable of automated assistance; and blanket exemption claims covering a mass of records are impermissible. For those reasons, Defendants are not absolved of their responsibility to produce the redacted free-text responses….
Production of the source material is essential for independent researchers to evaluate the vaccines and for medical professionals to provide meaningful treatment to their patients.…
Notably, Plaintiff points to several studies published and presented by CDC that rely upon on [sic] the V-safe data.... All but one of those studies considered only the first seven days after receiving a vaccine, and the only study that looked beyond the first week considered just two weeks.... Defendants do not contest this.... Rather, Defendants dismiss the limited scope of the published studies as just “the time period that some scientists have chosen to use in their research studies.” …
Because Defendants structured V-safe to collect health and symptomatic responses for a full year after a vaccine or booster, reviewing that data is of great importance to the public. If “some scientists” — sponsored or platformed by Defendants — “have chosen to use” only the first week or two of data to report the vaccine is safe and effective, then other scientists should be permitted to access the data to “pierce the veil of administrative secrecy,” “open agency action to the light of public scrutiny,” and “promote the disclosure of information.”… Many of the policies previously addressed were enacted because of guidance from Defendants. With billions of taxpayer dollars expended to develop, distribute, administer, and fund messaging campaigns, Plaintiff assumes a hefty and viable public interest in examining the raw clinical data. Production of the free-text data will permit independent researchers to put the government agencies to their proof by considering all of the available data.…
Additionally, Plaintiff marshalled evidence that some vaccine studies may be misleading or based upon cherry-picked data.... One study reported that 0.8% to 1.1% of users reported needing medical care according to the check-the-box data.... However, when the raw data was released pursuant to separate FOIA litigation, it showed some 7.7% of V-safe users reported needing medical care and an additional 25% missing school or work or unable to perform normal activities.... Similarly, Plaintiff alleges the check-the-box data captures only the “symptoms CDC says are normal to occur after vaccination and are actually a sign the vaccine is working.”… Thus, collecting that data and then profiling the vaccine as safe and effective based [sic] was a “pointless” exercise… Any concerning symptoms would necessarily be restricted to only the free-text responses, to date unexamined by independent researchers not sponsored by Defendants.
Finally, rapid vaccination of a huge percentage of the American population is nothing short of astounding, and the endeavor continues.… As of May 11, 2023, the CDC reports that more than 81% of Americans have received at least one dose, including nearly 32 million children. Understandably, there is substantial public interest in the data that supported, and continues to support, the government’s promotion of the COVID-19 vaccines and boosters. …
Plaintiff has shown an urgent need to inform the public about “actual or alleged Federal Government activity” — namely, related to the health and safety of the COVID-19 vaccines and policies.…
Additionally, as addressed above, Plaintiff presents evidence that calls into question the claim that the vaccines are safe and effective — or at least the scope of research supporting that claim....
Notably, the sample size is massive — representing between 3– 4.5% of the vaccinated population — thus permitting particularly accurate research. The V-safe free-text responses will contribute to the public’s understanding of the COVID-19 vaccines — specifically as to the assertion by Defendants, the Biden administration, and others that the vaccine is “safe and effective” for everyone over six months of age — by providing access to the direct source material to treating physicians, researchers, parents, recipients, and non-recipients.... “[D]isclosure of the information will” permit any interested person to research and report “whether CDC properly analyzed the information to detect and evaluate clinically important adverse events and safety issues that impacted its relevant policies or regulatory decisions and recommendations.” … Further, all Americans continue to be the target audience of marketing and messaging campaigns to promote continued vaccination. Additionally, even if the redacted responses are less useful than extrapolated MedDRA data, a position taken by Defendants…, the public nonetheless has the right to check the math.
CDC is ordered to comply with the following minimum production schedule:


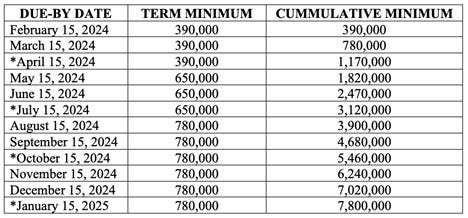
Sad that they could give you all this data on a thumb drive in an afternoon of effort. I think we can assume it will show myocarditis was an immediate signal. What will happen after this is all exposed?
Thank you so very, very much for your efforts, and for reporting this here. Having transcribed a large number of testimonies of people injured by the jabs 2021-2023, I can well imagine what is going to turn up in this data: a sickening avalanche of oftentimes extremely painful and debilitating cardiac, neurological, and immune issues.