What the “Casual Cruelty” of Dr. Paul Offit Reveals
Considered by many to be the world’s leading expert on “vaccine safety”
In response to a Twitter exchange I had with Dr. Paul Offit, he penned an article titled The Casual Cruelty of Placebo-Controlled Clinical Trials that makes numerous categorically false claims to argue against proper clinical trials for products injected into babies.
His article is deeply concerning because he is viewed by many as the leading medical authority on vaccine safety who, among other things, sits on the FDA’s vaccine committee that advises on whether to license childhood vaccines.
It is therefore worth reviewing every word of his article. But let’s first review the exchange leading up to it.
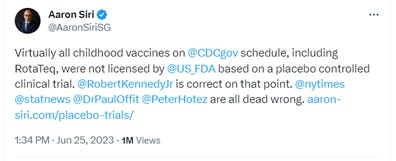
On June 25, 2023, in response to Offit’s claim that “all vaccines are tested in placebo-controlled trials before licensure,” I tweeted the following, linking an article with the proof – FDA sources – for my claim:
The next day, Offit responded with this:
I tweeted back later that same day:
By the next day, Offit quietly updated his original claim from “All vaccines…” to “Most vaccines are tested in placebo-controlled trials before licensure.” Offit’s updated claim is still categorically false, but we will return to that below.
Offit also, on July 1, 2023, responded to the above Tweet in a Substack article which, as noted above, is titled The Casual Cruelty of Placebo-Controlled Clinical Trials. His entire article is in bolded text below (you don’t want to miss a word) and, between paragraphs, I provide responses.
Offit’s article opens with this:
Anti-vaccine activists often tell the same story. Only the names of the vaccines, the materials in the vaccines, and the scientists who stand up for vaccines change. But the story remains the same. Government officials, pharmaceutical companies, public health agencies, scientists, and doctors are lying to you about vaccines. They are covering up safety problems. We, on the other hand, by pulling back the curtain on this conspiracy, will tell you the truth. Trust us. Not them.
An incredible opening. In our exchange, I made a statement about vaccines and then provided evidence to support that statement; I cited to FDA documentation for every routine childhood vaccine showing that virtually every single one lacked a placebo control. In response, Offit offers no proof to support his statement to the contrary or to refute my proof.
Instead, in a truly Machiavellian style, his opening paragraph tells you that if you don’t trust him, then you believe in some sort of conspiracy through which everyone else is “lying to you.” The lack of introspection is dumbfounding. When I and others, like Mr. Kennedy, raise concern about vaccine clinical trials we are not saying “trust us,” we are saying precisely the opposite: we are saying look at the proof yourself, here it is!
Offit, on the other hand, is not only saying “trust me,” while providing no evidence behind the supposed curtain he is pulling back, but he is also saying if you don’t trust him, you are an anti-vaccine conspiracy theorist. Let’s continue.
A recent iteration of this story was told on an episode of Joe Rogan’s podcast. RFK Jr. informed Rogan’s listeners that pharmaceutical companies “never do placebo-controlled trials.” Therefore, because what companies have called “placebos” during pre-licensure trials might have themselves been unsafe, we never really know whether vaccines are safe before licensure. This claim was recently supported by a lawyer [that’s me] working for an anti-vaccine group ICAN, which stands for Informed Consent Action Network. As the word “Informed” implies, only ICAN will really inform you about vaccines.
Offit is apparently not letting the facts get in his way. The claim at issue is about the critical safety testing that is supposed to occur pre-licensure and pre-injection into millions of babies. The reality – the hard, clear reality – is that not a single routine childhood vaccine was licensed based on a long-term placebo-controlled trial.
And I won’t name call you if you don’t agree, nor do I want you or anyone else to take my word for it which is why I cited to the proof for every single vaccine (citations repeated in the chart below). ICAN, an incredible organization that is dedicated to the truth and to “informed consent,” a phrase Offit seems unfamiliar with, does exactly the same by providing support for its assertions. Offit, still no proof. Just a “trust me.”
ICAN’s lawyer wrote, “Robert F. Kennedy, Jr. is on record stating that almost all childhood vaccines were licensed based on clinical trials that did not include a placebo control. [Kennedy] is correct. A placebo is defined by the CDC as a ‘substance or treatment that has no effect on living beings.’ This means a saline injection or water drops in mouth.”
Let’s take a closer look at the ICAN lawyer’s claims. First, the CDC doesn’t regulate vaccines; the FDA does. When researchers at pharmaceutical companies consider human testing, they immediately submit their plans to the FDA, which defines placebo as “inert,” meaning immunologically inactive and harmless. The FDA would not allow any vaccine trials to proceed unless they deemed placebos to be true placebos. (There is one exception, which we’ll get to later.) However, the ICAN lawyer’s claim that vaccine trials didn’t use a placebo control doesn’t mean that vaccines are unsafe or that they don’t work. Indeed, post-licensure studies comparing children who did or didn’t get vaccinated have consistently shown that vaccines are safe and effective.
Still no proof from Offit. More “just trust me.” Offit is now saying, without any proof and with his new definition of placebo, that (trust him) the FDA would never allow clinical trials for childhood vaccines without “true placebos.” But that is in fact precisely what the FDA has allowed! The proof again is the FDA’s own documentation – which I already cited to him and provide again below.
Amazingly, Offit then says that even if there were no placebo-controlled trials, there is no need to worry because “post-licensure studies comparing children who did or didn’t get vaccinated have consistently shown that vaccines are safe and effective.” But this claim is categorically false! Putting aside that Offit cites no studies for this claim, there is study after study after study finding that children without vaccines are healthier.
What makes Offit’s casual disregard for placebo-controlled trials so cruel is that, as he is no doubt aware, proving causation between a claimed injury and a vaccine without such a trial is extremely difficult, if not often impossible. (See Section I(iv) of this letter.) This is why Offit and his kin, in response to a claim of vaccine injury (outside of a few narrow exceptions they trot out to assure you vaccines are otherwise safe), will almost always tell you “it’s just correlation, not causation” or “it’s just anecdotal.” That is precisely why proper clinal trials are so important!
In fact, after licensure, pharma companies selling the vaccine (which cannot be sued for harms anyway) must, under federal law, disclose “only those adverse events for which there is some basis to believe there is a causal relationship between the drug and the occurrence of the adverse event.” Pursuant to this requirement, pharma companies have disclosed over 100 serious adverse harms they have a basis to assert are causally related to childhood vaccine products – see Appendix B. These are often the same harms parents complain of after vaccination! But without a clinical trial to prove causation, these parents (who, let’s remember, vaccinated their child) are called anti-vaxxers and lectured that “correlation does not equal causation” – like a religious mantra.
And everyone should be terribly concerned about this state of affairs because the chronic health of American children has taken a nosedive since the 1980s, which happens to be when manufacturers were given immunity to liability for vaccine injuries and the vaccine schedule grew from 2 routine injected vaccines totaling 7 injections, to 13 routine injected vaccines totaling 54 injections. (See Sections I and VII of this letter).
According to ICAN’s lawyer, the only substances that have “no effect on living beings” are water and salt water. Which is incorrect. Any chemical on this planet (both water and salt are chemicals) if given at a high enough dose, can be harmful. Drink 3-4 liters of water at one time, and you can suffer fatal water intoxication. Eat massive amounts of salt, and you can suffer fatal salt intoxication. In the words of Paracelsus, a 16th century physician, “All things are poisons; for there is nothing without poisonous qualities. It is only the dose which makes a thing poison.”
Hard to believe I need to explain this but when it comes to human beings, a saline injection is considered inert. Human beings are approximately 60% water and our blood is 0.9% salt by weight. Hence, saline solution (water with 0.9% salt) is used in clinical trials as a substance that has no effect on human beings. It also has a clearly defined safety profile and hence is often used as the control when conducting clinical trials of drugs (but not childhood vaccines).
While I prefer not to jest (given the seriousness of injecting babies with liability-free products), I guess I have to agree with Offit that, if someone tries to inject you with the full content of a syringe that is around three feet tall and half a foot wide (the size needed for 3-4 liters), sure, you should be concerned. It is telling that Offit needs to venture into the absurd to make his point. This is likely because, no doubt, Offit does not believe a saline injection used as a control would have an adverse effect on living beings.
ICAN’s lawyer argued that the only true placebos were water or saltwater, which isn’t true. Indeed, a wide range of placebos have been used in vaccine trials. These placebos might contain buffers, stabilizing agents, emulsifying agents, or adjuvants, like aluminum salts. They might contain sodium citrate, sodium phosphate, sucrose, or polysorbate-80. At the level contained in vaccines, all these chemicals are safe, including aluminum salts. Therefore, all meet the FDA criteria for a placebo.
First, most clinical trials of childhood vaccines use another vaccine as a control – see chart below – which even Offit must agree cannot be considered a placebo (because Offit’s own definition required that a “placebo,” among other things, be “immunologically inactive”).
Second, when something other than a vaccine is used as a control, it often includes, as Offit points out, numerous ingredients that are entirely unnecessary! This means they are not a placebo! An injection of saline solution is a placebo. An injection of aluminum salts, which is an adjuvant used for the very purpose of generating a strong immune response, is not a placebo under any definition of the term (again, including Offit’s own definition). Below we will review the actual control used in the trials for each childhood vaccine – based on the actual evidence from the FDA – and you will see with your own eyes that virtually every single one fails to meet even Offit’s definition.
Not all vaccine trials, however, are placebo controlled. As noted by ICAN’s lawyer: “Prevnar-13 was licensed based on a trial comparing it to Prevnar-7.” Prevnar-7, which was licensed in the United States in 2000, was designed to prevent seven of the most common types of pneumococci that cause pneumonia, meningitis, and bloodstream infections (sepsis), collectively referred to as invasive pneumococcal disease. Prior to the availability of Prevnar-7, pneumococci caused about 17,000 cases of invasive disease, 700 cases of meningitis, and 200 deaths in children less than 5 years of age every year. Prevnar-7 worked, clearly reducing the incidence of invasive disease. To further broaden protection, researchers developed Prevnar-13, which protected against an additional six types. ICAN’s lawyer apparently believes it would have been ethical to study Prevnar-13 pre-licensure with a water or saltwater placebo, knowing that a vaccine already existed that offered considerable protection against a severe and occasionally fatal bacterial infection. I can’t imagine how this kind of trial would have been explained to parents. Not surprisingly, according to a World Health Organization Advisory Panel, the study proposed by ICAN’s lawyer would have been unethical.
Offit does not address what I actually wrote about Prevnar. What I wrote was that “use of vaccines as controls is highly concerning because the control vaccines were virtually never licensed based on a placebo-controlled trial … [and this] is true even for trials of the first vaccine for a target disease. … For example, Prevnar-13 was licensed based on a trial comparing it to Prevnar-7, and Prevnar-7 was licensed based on a trial comparing it to another experimental vaccine.” (emphasis added).
My point, as I clearly wrote, was that because Prevnar-7 was the first licensed vaccine of its kind in the United States, there was absolutely no excuse not to trial it against a placebo. Certainly, there was no excuse to trial it against another experimental vaccine! Offit has no response to that point because there is no excuse for such a morally and ethically bankrupt clinical trial.
Here is where it gets more than casually cruel. All one can say about Prevnar 7 is that it was at best shown to be equally as “safe” as another experimental vaccine. Meaning both could be terribly unsafe. And to that point, as I pointed out to Offit, when Prevnar 7 was then used as the control in the clinical trial for Prevnar 13, here is what occurred: “Serious adverse events reported following vaccination in infants and toddlers occurred in 8.2% among Prevnar 13 recipients and 7.2% among Prevnar 7 recipients.” Meaning, as I wrote in my tweet to him above, “these two vaccines were equally safe by FDA standards – but equally unsafe by any other standard.” His response to that? None, because this finding is viciously cruel.
Indeed, just take a look at the FDA’s definition of “serious adverse event” – it means death, life-threatening, hospitalization, disability or permanent damage, congenital anomaly/birth defect, intervention to prevent permanent impairment or damage, or other serious medical event consistent with these. Meaning “serious adverse event” is something very serious! It is not normal for a group of healthy children to suffer this rate of serious adverse events every six months, absent something harming them! It is viciously cruel to ignore this data. Prevnar 7 was never properly assessed as safe in a clinical trial and, hence, using it as the baseline of safety for Prevnar 13 is morally and ethically bankrupt.
Instead of addressing this serious safety concern, Offit ignores it in his response. Instead, he sets up a false argument to bat down, and finally tries to focus on the efficacy of this product, meaning its ability to prevent disease. But even if the product is highly effective, unless you know the actual safety profile, you cannot know if you are causing more harm than good. It is also impossible to obtain informed consent and to allow parents and providers to make a good medical decision for each individual child.
The CDC recommends injecting this product three times during the first six months of life to the over 4 million babies born in the United States each year. If this product causes a serious adverse event in just 1% of these infants (let alone 8.2% as occurred in the clinical trial), that would mean serious harm to over 40,000 babies a year from this product. This could certainly far exceed the claimed benefit. But this type of guesswork is no way to practice medicine – what is needed is a proper clinical trial so that the actual safety profile of this product is known.
And Offit’s purported ethical concern is rich. Putting this into sharp focus, in the study that Offit cites regarding Prevnar 7 (in which it was trialed against another experimental vaccine), 15 children died after getting Prevnar 7, and 7 died after getting the other experimental vaccine (including sudden infant deaths, simply stopping to breathe, as well as accidents that could result from fainting or seizures caused by vaccination). But rest assured: the study says that none of the deaths were “judged [by Pfizer’s paid researchers] to be associated with vaccine.” This morally bankrupt study, comparing two experimental vaccines, relies upon the “judgment” of the company that stands to earn billions to make decisions on causality! It should simply be a valid statistical comparison of the rate of death between a group receiving Prevnar 7 and a group receiving a placebo. That didn’t happen and will now never happen, and with Pfizer now making literally billions of dollars annually through sales of this product, good luck overcoming that juggernaut to get to the truth. But as Offit already told you – if you don’t just trust him on this, you are a conspiracy theorist.
And here is how Offit’s article ends.
The casual cruelty expressed by ICAN’s lawyer can also be found in an event that occurred almost 70 years ago. In 1954, 420,000 first and second graders in the United States were inoculated with Jonas Salk’s inactivated polio vaccine; 200,000 were inoculated with salt water. It was one of the largest placebo-controlled trials of a medical product in history. Jonas Salk didn’t want to do it. He couldn’t conscience giving a saltwater shot to young children when as many as 50,000 were paralyzed by polio and 1,500 died every year. When the trial was over, the vaccine was declared “safe, effective, and potent.” Church bells rang out; synagogues held special prayer meetings; department store patrons stopped to listen to the results of the trial over loudspeakers. How did we know that Jonas Salk’s polio vaccine was effective? We knew because 16 children died from polio in that study—all in the placebo group. We knew because 34 of the 36 children paralyzed by polio in that study were in the placebo group. These are the gentle heroes we leave behind.
I suspect that none of the parents who volunteered for Jonas Salk’s polio vaccine trial were hoping their children were in the placebo group.
More false claims by Offit. First, it is categorically false to claim that “200,000 were inoculated with salt water.” These children did not receive “salt water.” The official final report from the Salk trial (Dr. Offit, I am happy to send you a copy), on page 51, describes precisely what these 200,000 children received as a control. It was an injection that included, among other things, the following ingredients: “199 solution” (a synthetic tissue culture medium and ethanol) “phenol red,” “antibiotics,” and “formalin.” Don’t take my word for it, see the official report for yourself. It is categorically false for Offit to claim “200,000 were inoculated with salt water.”
And keep in mind, this is the one and only vaccine in his article he actually identified as being licensed based on a placebo control - a saline injection!
Offit then claims that “16 children died … all in the placebo group” and that “34 of the 36 children paralyzed by polio in that study were in the placebo group.” But the article he cites for this claim says, “There were four deaths among children who received placebo” and that “33 inoculated children receiving the complete vaccination series became paralyzed.”
Offit also fails to mention the Salk vaccine ceased being used in the 1960s and does not disclose the many paradoxical and statistically improbable realities regarding the rise of polio and the claim that Salk’s vaccine vanquished this disease. But again, that also misses the point. The point is that whatever the efficacy, the actual safety profile of each routine vaccine product on the CDC schedule needs to be determined in a valid clinical trial. Without that, you can never provide any parent proper informed consent.
Offit kindly calls children that died of polio during the Salk trial “gentle heroes.” They are. Every child is precious. We should care about every child injured by an infectious disease. The issue is that when children are injured or killed by a vaccine, they are not called “gentle heroes.” Instead, their parents are called pejoratives, gaslit, cast out of medical offices, and berated and demeaned in an attempt to ignore what they experienced.
If refusing them proper medical care and adding insult to injury were not enough, they are then attacked by their own government if they happen to learn about the Vaccine Injury Compensation Program (which most do not know about) and file a claim (most do not because they miss the time to file) because they must fight against an army at HHS (the department in which CDC and FDA are located) and DOJ attorneys in order to seek even minimal compensation to care for their injured children. This is a system that is rigged against the vaccine injured.
So, why is Offit’s article incredible? It is incredible because he is literally the person the medical community and the FDA and CDC look to on the issue of vaccine safety. Offit is maybe the most renowned disciple of the godfather of vaccines, Dr. Stanly Plotkin, and one of the four editors of the medical textbook Plotkin’s Vaccines and the author of the chapter “Vaccine Safety” in this textbook, hailed “the bible of vaccinology.” He is also the Director of the Vaccine Education Center at CHOP which, I can tell you from deposing numerous pediatricians, is looked to as the pinnacle of truth regarding vaccine safety. He is even a current member of the FDA’s vaccine advisory committee and a former member of the CDC’s vaccine advisory committee.
Yet his article above shows not merely a casual disregard for proper clinical trials of childhood vaccines but in fact contempt for such trials – trials that would actually demonstrate the true safety profile of these products.
While Offit called me casually cruel for asking for placebo-controlled trials for vaccines where no vaccine exists for the target disease or when the control used was never validated through a prior placebo-controlled trial, what he was really doing was revealing more about himself; just as he sees the hero in children injured by infectious disease and expresses a deep caring for them, as we all should, he reveals a deep contempt for children injured by vaccines and, worse, for those seeking to identify and avoid such injuries in other children. That is more than casually cruel. And that is being generous.
If Offit can show that, in fact, all the routine vaccines on the childhood vaccine schedule were originally licensed based on a properly designed placebo-controlled trial (even using his narrow definition) then I will retract my assertion. I would in fact welcome being wrong. Like probably every other parent, there would be a lot of comfort in knowing that the products he pushes for injection into babies dozens of times in the first six months of life were licensed based on proper trials. Here is the chart with the proof that this is not, unfortunately, what occurs with childhood vaccines. I welcome receiving from you, Dr. Offit, proof to the contrary.
One final note: Offit wrote (regarding Mr. Kennedy) that, “You can’t debate someone who knowingly manipulates or flat out lies about the facts to advance their claims.” Our debate to date shows that Offit is incorrect. Many would submit that Offit’s article reflects “someone who knowingly manipulates or flat out lies about the facts to advance their claims” but, yet, Offit and I have engaged in what many would consider a respectful debate based on evidence.
Proof Regarding the Clinical Trials Relied Upon by the FDA to License the Childhood Vaccines on the CDC Childhood Vaccine Schedule
HepB vaccine (Birth 1M 6M)
Recombivax HB (Merck) licensed for babies based on trials with no placebo control & 5 days of safety monitoring after injection. See Package insert § 6.1
Engerix B (GSK) licensed for babies based on trials with no placebo control & 4 days of safety monitoring after injection. See Package insert § 6.1
DTaP vaccine (2M 4M 6M 15M 4Y)
Infanrix (GSK) licensed for babies based on trials with no placebo control (DTP vaccine used as a control) & up to 30 days of safety review after injection. See Package insert § 6.1 (Note that DTP was not licensed in a placebo-controlled trial and increases mortality)
Daptacel (Sanofi) licensed for babies based on trials with no placebo control (DT or DTP vaccine used as control) & 2 months of safety review after injection except one trial which was 6 months with no control, 1,454 children and “[w]ithin 30 days following any dose of DAPTACEL, 3.9% subjects reported at least one serious adverse event.” See Package insert § 6.1 (see note for Infanrix)
PCV vaccine (2M 4M 6M 12M)
Prevnar 13, PCV-13 (Wyeth, part of Pfizer) licensed for babies based on trials with no placebo control (Prevnar 7 used as a control, and Prevnar 7 was licensed based on trial in which the control was another experimental vaccine) & 6 months of safety review after injection which found, “Serious adverse events reported following vaccination in infants and toddlers occurred in 8.2% among Prevnar 13 recipients and 7.2% among Prevnar 7 recipients.” See Package insert § 6.1 (Note the package insert for Prevnar 7 states the control in its licensing trial was an “Investigational meningococcal group C conjugate vaccine”)
Vaxneuvance PCV-15 (Merck) licensed for babies based on trials with no placebo control (Prevnar 13 used as the control) & up to 6 months of safety review after injection finding that, “Among children who received VAXNEUVANCE (N=3,349) or Prevnar 13 (N=1,814) … serious adverse events up to 6 months following vaccination with the 4-dose series were reported by 9.6% of VAXNEUVANCE recipients and by 8.9% of Prevnar 13 recipients.” Deemed “safe” because, “[t]here were no notable patterns or numerical imbalances between vaccination groups.” See Package insert § 6.1
Prevnar 20, PCV-20 (Pfizer) licensed for babies based on trials with no placebo control (Prevnar 13 was used as the control) & up to 6 months of safety review after injection that again showed high rates of serious events (this time broken up into two categories – “serious adverse events (SAEs)” and “newly diagnosed chronic medical conditions (NDCMCs)”) in both vaccine groups but deemed “safe” because “no notable patterns or imbalances between vaccine groups.” See Package insert § 6.1; Clinical Review
Polio vaccine (2M 4M 6M 4Y)
IPOL (Sanofi) licensed in 1990 for babies based on trials with no placebo control & 3 days of safety review after injection. Sanofi reports that, “Although no causal relationship has been established, deaths have occurred in temporal association after vaccination of infants with IPV.” See Package insert at 14-17 (Note that IPOL is an injected polio vaccine and is the only polio vaccine used in the U.S. for over two decades. It is a very different product than the polio vaccine developed by Salk in the 1950s – and, as noted above, ceased being used in the U.S. in the 1960s – and hence the trials of Salk’s vaccine from the early 1950s were not relied upon to license IPOL.)
Hib vaccine (2M 4M 6M 12M)
ActHIB (Sanofi) licensed for babies based on trials with no placebo control (Hepatitis B vaccine used as control) & 30 days of safety review after injection during which 3.4% experienced a serious adverse event but “[n]one was assessed by the investigators [Sonafi] as related to the study of vaccines.” See Package insert § 6.1; Basis of Approval at 8
Hiberix (GSK) licensed for babies based on trials with no placebo control (Unlicensed Hib vaccines and HibTITER used as the control) & 31 days of safety review after injection. See Package insert § 6.1; Clinical review at 20-21
Liquid PedvaxHIB (Merck) licensed for babies based on trials with no placebo control (Lyophilized PedvaxHIB used a control) & 3 days of safety review after injection. See Package insert at 6-8 (Note that Lyophilized PedvaxHIB was tested in a trial in which controls were given placebo, OPV and DTP but there is no indication Lyophilized PedvaxHIB was ever licensed)
Rotavirus vaccine (2M 4M 6M) (Note that every vaccine on the CDC childhood schedule is given via injection, except for one flu vaccine given by nasal spray and the rotavirus vaccines, which are given by oral drops in the mouth.)
Rotarix (GSK) licensed for babies based on trials without a placebo control (the control group received an oral drop that included Dextran, Sorbitol, Amino Acids, Dulbecco’s Modified Eagle Medium, and Xanthan) & 31 days of safety review after oral dose and up to a year in some trials for cases of intussusception. There were more deaths in the group receiving Rotarix than the purported placebo. As disclosed by the FDA and GSK: “During the entire course of 8 clinical studies (Studies 1 to 8), there were 68 (0.19%) deaths following administration of ROTARIX (n = 36,755) and 50 (0.15%) deaths following placebo administration (n = 34,454). The most commonly reported cause of death following vaccination was pneumonia, which was observed in 19 (0.05%) recipients of ROTARIX and 10 (0.03%) placebo recipients (RR: 1.74, 95% CI: 0.76, 4.23).” See Package insert § 6.1 (claims used a placebo); Clinical review at 23-24 (admits the purported “placebo” included all the foregoing ingredients)
RotaTeq (Merck) licensed for babies based on trials without a placebo control (the control group received an oral drop that included Polysorbate-80, Tissue Culture Medium, Fetal Bovine Serum, and Sodium Phosphate) & 42 days of safety review after each oral dose and up to a year for cases of intussusception. See Package insert § 6.1 (claims used placebo); Clinical reports at 445 etc. (admits the purported “placebo” included all the foregoing ingredients)
Covid-19 vaccine (6M 7M 10M)
Comirnaty (Pfizer) licensed for babies based on trial with a placebo control (finally!) & 6 months of safety review after injection. Package insert § 6.1 (Note that Comirnaty is currently only licensed for 12 years and older and Spikevax, Moderna, is only licensed for 18 years and older. Also, while Comirnaty’s trial had a placebo control group, that group was unblinded and most were vaccinated during the 6-month safety review period. The 16- and 17-year-old data is not separated from the adult data, but the 12- to 15-year-old data is separated and included only 1,131 children who received a vaccine, and the case of one participant reflects how this trial was conducted.)
Flu vaccine (6M 7M Annually)
The formulation for each influenza vaccine changes annually and there is no clinical trial carried out for each new formulation. (In any event, none of the clinical trials for the original formulation of any injected influenza vaccine for children had a placebo control group, see letter pp.13-14, even though some adult trial did, showing it could have been done, see FDA documentation and compare child and adult portions of Section 6.1 of each flu vaccine package insert. The one inhaled influenza vaccine’s original trial had a placebo but, again, its formulation changes every year and is not safety tested in any trial.)
MMR vaccine (12M 4Y)
M-M-R-II (Merck) licensed based on a trial with no placebo control & 42 days of safety review after injection in a trial with a total of only 834 children of which a third developed gastrointestinal issues and a third respiratory issues. See Clinical reports (This patently deficient underpowered, unblinded, and non-randomized trial is unsurprisingly not even listed in the safety section of M-M-R-II’s package insert. Also note that the original MMR’s clinical trial was similarly deficient and also showed a high and concerning rate of gastrointestinal, respiratory and other issues, as compared to the small untreated control group – see pages 12 and 13. In any event, the original MMR was a different product that did not include millions of pieces of human DNA and cellular debris, as does M-M-R-II, which is likely why it was not used as a control in the trial for M-M-R-II).
Priorix (GSK) licensed based on trials with no placebo control (M-M-R-II used as the control) & 6 months of safety review after injection in which both vaccine groups had a high rate of serious adverse events, emergency room visits, and new onset of chronic diseases (e.g., autoimmune disorders, asthma, type I diabetes, vasculitis, celiac disease, thrombocytopenia, and allergies), see second link at page 12. See Package insert § 6.1; Sup materials at 12
Varicella (chicken pox) vaccine (12M 4Y)
Varivax (Merck) licensed based on trials with no placebo control (the purported “placebo” was actually an injection of 45 mg of neomycin per milliliter) & 70 days of safety review after injection which included only one controlled trial of 956 children in which around half received Varivax and half received the injection of 45 mg of neomycin per milliliter, and there was one trial in which 32 children received Varivax and 29 children received nothing and then received Varivax eight weeks later; during this eight-week period, the Varivax group had double the rate of ear infection and a 50% increase in respiratory infection. As for serious adverse events, Merck did not consider any related to Varivax. See Package insert § 6.1; Merck study at 2; Clinical reports
HepA vaccine (12M 18M)
Havrix (GSK) licensed based on trials with no placebo control (Engerix-B was used as a control) & 31 days of safety review after injection with a phone call follow-up at 6 months. Package insert § 6.1
Vaqta (Merck) licensed based on trials with no placebo control (an injection of AAHS, an aluminum adjuvant, and thimerosal, a form of mercury, were used as a control) & up to 42 days of safety review after injection. Package insert § 6.1 (using term “placebo”); Merck study at 454 (admits the purported “placebo” included all the foregoing ingredients) (Note that trials for Havrix and Vaqta occurred at roughly the same time and, because there was no licensed Hepatitis A vaccine at that time, there was no excuse for not using a placebo control in these trials. It is also startling that Engerix-B, which had 4 days of safety monitoring in its trial, was used as the control for Havrix, and that an injection of known cyto-and-neuro toxic substances, AAHS and thimerosal, were used as a control for Vaqta instead of just a saline injection.)
Tdap vaccine (11Y)
Adacel (Sanofi) licensed based on trials with no placebo control (Td, for adult use, was used as a control) & up to 6 months of safety review after injection. See Package insert § 6.1
Boostrix (GSK) licensed based on trials with no placebo control (DECAVAC or Adacel was used as a control) & up to 6 months of safety review after injection. See Package insert § 6.1
HPV vaccine (9Y 9 ½Y)
Gardasil 9 (Merck) was licensed based on trials in which safety was reviewed after injection for 1 month in five of the clinical trials, 6 months in a lot consistency trial, and 4 years in one trial of women aged 16 to 26 years (reflecting that a safety trial of a more appropriate duration is possible). These Gardasil 9 trials were either not controlled or used Gardasil 4 as the control except for one trial in which 306 participants received a placebo but only after receiving the full series of Gardasil 4 injections. See Clinical review at 17-19 (Note that in Gardasil 4’s clinical trial, controls received aluminum adjuvant, AAHS, except 320 people labeled “Saline Placebo” that actually received all vaccine ingredients except antigens and AAHS. Also, across all these trials, 2-3% of participants receiving vaccine or aluminum adjuvant -- used to induce autoimmunity -- had a suspected autoimmune disorder.)
Men4 vaccine (11Y 16Y)
Menactra (Sanofi) licensed based on trials with no placebo control (Menomune used as the control, and amazingly the safety section of the package insert for Menomune lists this same trial in which it is being used as a control) & up to 6 months of safety review after injection. See Package insert § 6.1
Menveo (GSK) licensed based on trials with no placebo control (Menactra, Boostrix, or other vaccines used as a control) & up to 6 months of safety review after injection. Package insert § 6.1
MenQuadfi (Sanofi) licensed based on trials with no placebo control (Menveo or other vaccines used as a control) & up to 6 months of safety review after injection. Package insert § 6.1 (The three Men4 vaccines provides another good example of the vaccine safety pyramid scheme because Menomune was licensed without a placebo-controlled trial and then used as the control to license Menactra; Menactra is then used as the control to license Menveo; and then Menveo is used as the control to license MenQuadfi. The actual safety profile, putting aside the limited 6-month safety period, is unknown since Menomune’s safety baseline was never established in a placebo-controlled trial.)
MenB vaccine (10Y+ if indicated)
Bexsero (GSK) licensed based on trials with no placebo control group (either uncontrolled or control group was given an injection of aluminum hydroxide and, in one trial involving 120 adolescents, a saline injection followed by an injection of Menveo and hence FDA labels this an “active control” and not a “placebo control” trial) & 30 days of safety review after injection. See Summary basis at 14-15; Clinical review at 40
Trumenba (Pfizer) licensed based on trials with no placebo control group other than 12 people in a dose ranging phase II study (otherwise the controls were injection of Gardasil+placebo, dTaP-IPV+placebo, HepA+placebo, or Menactra+Adacel+placebo) & 30 days of safety review after injection for one of the three trials and up to 11 months in the other two trials. See Summary basis at 4; Clinical review at 9-10
PPSV23 vaccine (2Y+ if indicated)
Pneumovax 23 (Merck) is licensed for children 2 years and older but there is no indication that there was any clinical trial involving anyone younger than 16 years of age that the FDA relied upon to license this vaccine. See FDA documentation
Dengue vaccine (6Y+ if previously had dengue and live in area dengue is endemic)
Dengvaxia (Sanofi) licensed based on a trial with 11,474 children receiving a placebo control (saline injection) & 5 years of safety review after injection. Meaning, the 17th and last vaccine on the CDC’s childhood vaccine schedule, is apparently the first vaccine that underwent a longer-term placebo-controlled trial prior to licensure! This trial stands as the proof that a longer-term placebo-controlled trial of a childhood vaccine is possible! See Statistical review at 10; Package insert at 4 (Note for this vaccine, it was learned that children under 6 years old had an increased risk of severe harm and death from this vaccine – harm that would likely never be uncovered by the trials performed for any of the other 16 vaccines. It was also found that children older than 6 who had never had dengue and received this vaccine likewise had a seriously increased risk of severe harm and death. Hence, this vaccine is only to be given to older children who have previously had dengue. As disclosed by the FDA and Sanofi: “Those not previously infected are at increased risk for severe dengue disease when vaccinated and subsequently infected with dengue virus.” This vaccine is only recommended for children in endemic dengue areas and dengue is not endemic in the United States.)

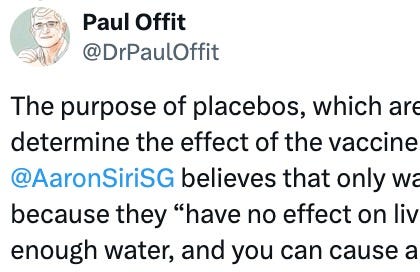




It is sad to see Paul Offit make such a fool of himself on this twitter exchange. Thanks Aaron for the valuable work you do in exposing this fraud.
They can do all the clinical trials they want; I will never under any circumstances ever take any vaccine.